
Answering clinical questions requires high quality evidence, so levels of evidence (or hierarchies of evidence) and research study designs are used in EBP to help identify the best possible evidence easily.
Different types of clinical questions will require different levels of primary evidence to find appropriate, reliable answers.
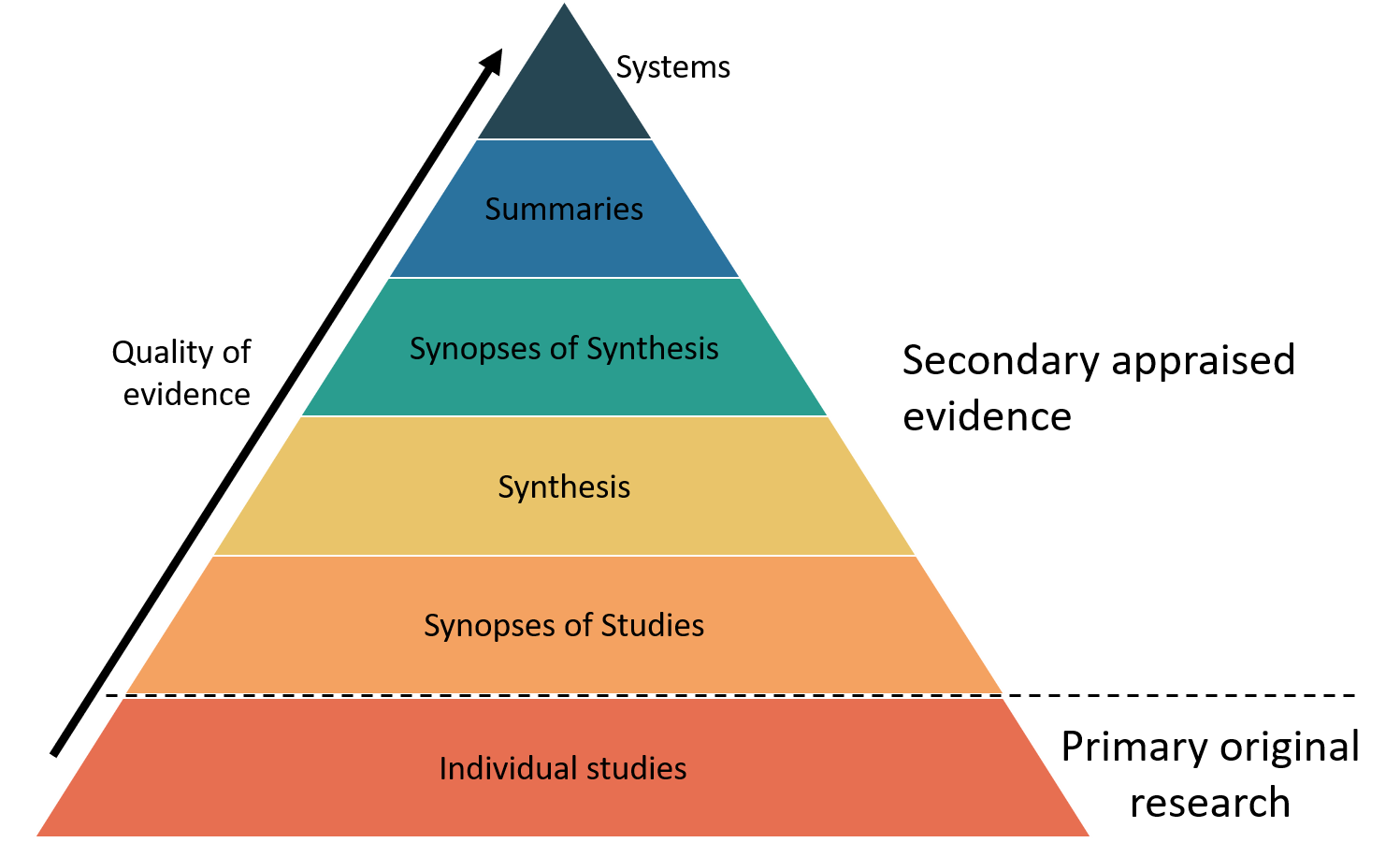 The 6 S levels of evidence (shown in these diagrams) show that secondary sources that have appraised and synthesised many individual studies are considered higher quality sources, compared to individual research studies (DiCesno, Bayley & Haynes, 2009; ehealth NSW, 2018). The highest level of evidence available should be what you use to answer clinical questions.
The 6 S levels of evidence (shown in these diagrams) show that secondary sources that have appraised and synthesised many individual studies are considered higher quality sources, compared to individual research studies (DiCesno, Bayley & Haynes, 2009; ehealth NSW, 2018). The highest level of evidence available should be what you use to answer clinical questions.
When individual studies are used, the research design of the studies is important to understand. Using higher quality research designs helps reduce bias, and increase the reliability of research, which helps support clinical decision making. However, many research questions can't be answered with study designs like a Randomised Control Trial (RCT), so the type of question you are asking must be taken into account.
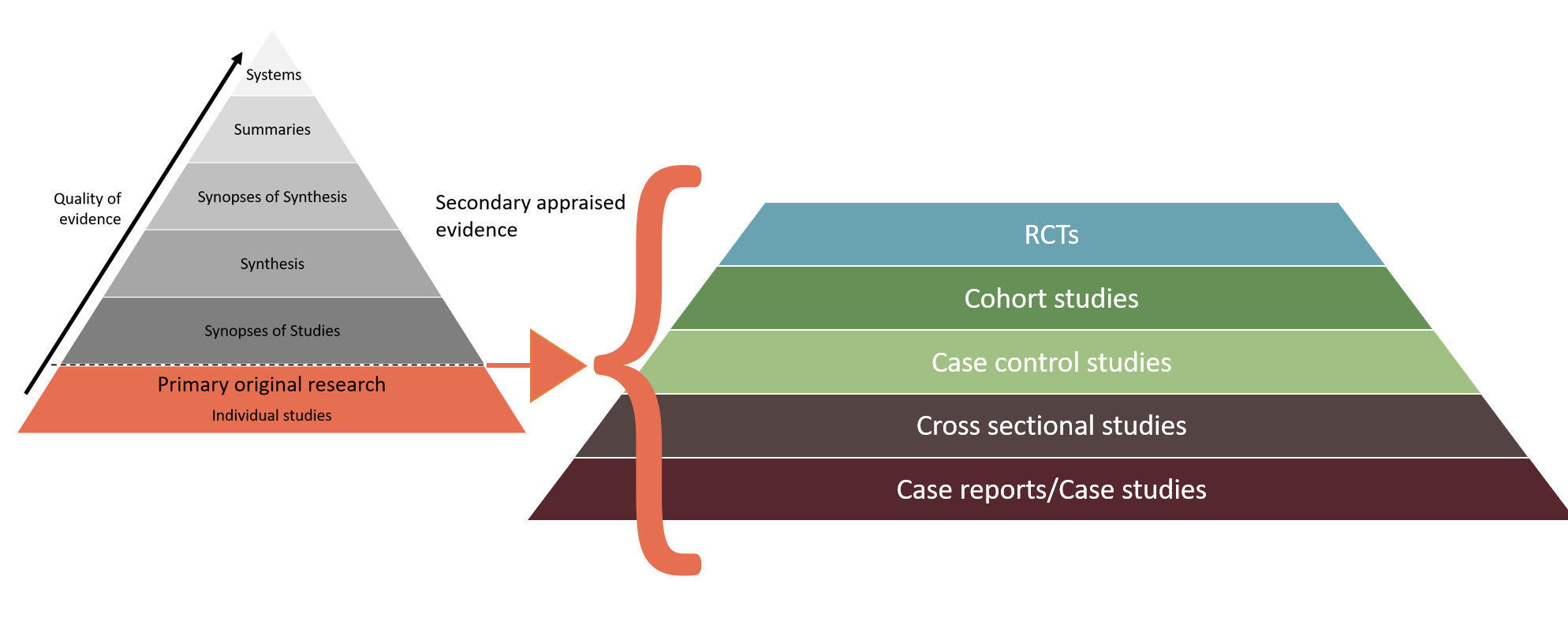
Diagrams adapted from eHealth NSW (2018).
For more information about the levels of evidence, check the Introduction to Evidence-Based Practice and CIAP module from NSW Health.
Understanding the type of clinical question, you are asking and the appropriate research methodologies to answer it will also help you decide where to search for evidence, and how to appraise the evidence.
| Type of question | Explanation | Types of evidence to answer the question |
|---|---|---|
| Therapy (treatment) | Questions about the effectiveness of interventions in improving outcomes for patients. Includes medications, surgeries, or service delivery | Randomised Controlled Trial (RCT) |
| Prevention | Questions about reducing the chance of disease, identifying risk factors or screening | RCT or Prospective study |
| Diagnosis | Questions about the validity of diagnostic or screening tests | RCT or Cohort Study or Cross-sectional study |
| Prognosis | Questions about the likely course of a condition | Cohort Study or Case-Control Series or Longitudinal study |
| Etiology (causation) | Questions about determining if a harmful factor is related to the development or course of a condition | Cohort Study |
| Meaning | Questions about the patient experience | Qualitative Study |
DiCesno, A., Bayley, L., & Haynes, B. (2009). Accessing pre-appraised evidence: fine-tuning the 5S model into a 6S model. Evidence Based Nursing, 12(4). https://doi.org/10.1136/ebn.12.4.99-b
eHealth NSW. (2018). Clinical Information Access Portal (CIAP) EBP Learning Modules. https://www.ciap.health.nsw.gov.au/training/ebp-learning-modules/module1/index.html
Bushnell University Kellenberger Library | 828 E. 11th St., Eugene, Oregon 97401 | (541) 684-7233 | librarian@bushnell.edu